World’s First Phase III Clinical Trial of COVID
Inactivated Vaccine Begins in UAE
The first WHO enlisted global clinical Phase III trial of
Sinopharm CNBG’s inactivated vaccine to combat COVID-19
has started in Abu Dhabi, inspired by the UAE Leadership’s
commitment to overcome the pandemic through a global
collaborative effort.
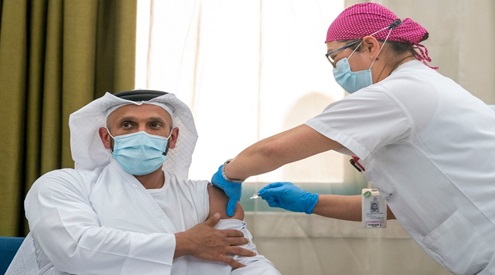
H.E. Sheikh Abdullah bin Mohammed Al Hamed, Chairman
of the Department of Health, Abu Dhabi, was the first in the
world to commence the trial of a Phase III inactivated
vaccine for COVID-19 that is the result of a cooperation
partnership between Abu Dhabi based G42 Healthcare and
Sinopharm CNBG, the world’s sixth largest vaccine
manufacturer.
The UAE was selected to conduct the trials as the nation is
home to over 200 nationalities, allowing for robust research
across multiple ethnicities and increasing its feasibility for
global application. They are being operated by Abu Dhabi
Health Services (SEHA) who are providing clinical facilities.
UAE Health Authorities have issued a permit for up to
15,000 volunteers to participate and G42 Healthcare and
SEHA are working towards achieving a minimum of 5,000
participants for the trials being supervised by the
Department of Health Abu Dhabi and SEHA following
international guidelines stipulated by WHO and USFDA.
Chairperson of the UAE National COVID-19 Clinical
Management Committee Dr. Nawal Ahmed Alkaabi said:
“Our participation in this trial enables us to make a major
contribution in the global fight to combat the COVID-19
pandemic. We are proud to facilitate the testing process
that if successful leads to the manufacture of a vaccine.”
It follows successful phase I and II trials by Sinopharm in
China, resulting in 100% of volunteers generating antibodies.
G42 Healthcare CEO Ashish Koshy said: “We are
enormously proud to partner with Sinopharm CNBG in this
groundbreaking phase III clinical trial. Using our AI
solutions, super-computer, advanced diagnostics solutions
for COVID-19, and G42 Healthcare will be responsible for
running clinical operations for these trials.”
Jingjin Zhu, President, Biological products, Sinopharm
CNBG added: “The UAE is a nation of innovation and
tolerance, home to individuals from every part of the world
and ethnic background. We will work closely with our
partner G42 Healthcare, with the support of the Abu Dhabi
health authorities to complete these clinical trials
successfully and make this vaccine available to people in
need worldwide.”

 التكنولوجيا وأخبارها بوابة الإمارات لتكنولوجيا المعلومات والإتصالات
التكنولوجيا وأخبارها بوابة الإمارات لتكنولوجيا المعلومات والإتصالات